COVID-19 Vaccine Hesitancy and Education: Now is the the Time
Dr. Leslie Eber put together a presentation addressing many of the concerns surrounding the new COVID-19 vaccine. The presentation address questions such as
- Is it Safe
- Why should we trust the vaccine?
- Is there new technology being used and is that dangerous?
- What is an EUA and what does that mean for me?
- When and how long will I be protected?
- Will I still need to wear a mask?
- Expectations: Possible side effects?
- Special circumstances: What if I had COVID 19 or if I have antibodies?
This page is adapted from Dr. Ebers powerpoint presented 11/25 at the CHCA Bi-Weekly Facility call. Click here to view a PDF of the original presentation
COVID-19 Vaccine Hesitation is real
Kerps et alfound in his survey published in JAMA 10/20/20 that the most important factors for acceptance are efficacy, duration of protection and lower incidence of major side effects (source)
Specific LTC staff concerns include:
- “being first”
- Safety
- Not being represented in the vaccine trials
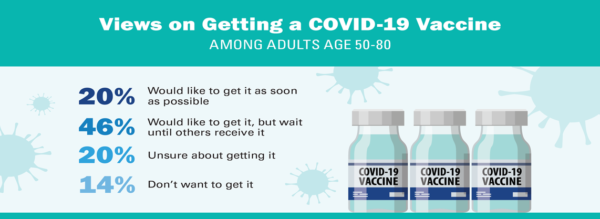
COVID 19 Hesitancy and Older Adults
National Poll on Healthy Aging report, University of Michigan (November, 2020)
(source)
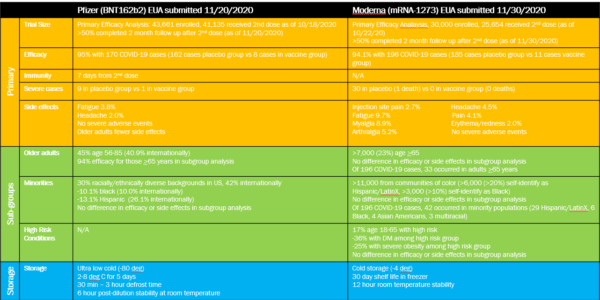
Dr. Anuj Metha Notes: More information is constantly becoming available. Sub-group comparisons (e.g. comparisons about efficacy between races or age groups) may be less accurate due to smaller numbers. Sub-group numbers for the Pfizer vaccine are given for US participants with international percentages in parentheses.
(source1) (source 2) (source 3) (source 4)
Astrazeneca (AZD1222)
- Inactivated adenovirus vector with spike protein expression
- Cheaper and easier to manufacture. Easier storage. 6 month shelf life.
- Interim analysis 11,636 participants pooled 70% efficacy (131 cases)
- 2,741 participants half dose/full dose vs placebo arm (~90% effective) almost no participants >55 years
- 8,895 participants full dose/full dose vs placebo arm (~60% effective)
- “No serious adverse events”
- Target 23,000 COV002 (Phase II/III in UK) and COV003 (Phase III in Brazil) – trials had different protocols but press release results are pooled analysis
- Goal to enroll 60,000 more
Courtesy of Dr. Anuj Mehta, 11/30/20
A Strategy for COVID-19 Education
Common questions from staff might include:
- Is it Safe
- Why should we trust the vaccine?
- Is there new technology being used and is that dangerous?
- What is an EUA and what does that mean for me?
- When and how long will I be protected?
- Will I still need to wear a mask?
- Expectations: Possible side effects?
- Special circumstances: What if I had COVID 19 or if I have antibodies?
Are the COVID 19 Vaccines Safe?
Safety is the most important priority in vaccine approval
Most adverse side effects occur within 6 weeks of vaccine administration, and the FDA has required 8 weeks of safety monitoring
FDA advises a minimum of 3,000 participants to assess safety. The current phase 3 trials have 30,000 to 50,000 participants. This really demonstrates how safety is a top priority for the FDA and the medical community.
Why should we trust the COVID 19 Vaccine
- The FDA is using the same standards that it has for decades
- There are no steps being “skipped”
- 2 advisory committees:
- The Vaccine and Related Biological Products Advisory Committee (VRBPAC) that advises the FDA
- The Advisory Committee on Immunization Practices (ACIP) that advises the CDC.
New Technology for the COVID 19 Vaccine
Question: Can these new technologies give me COVID 19?
Answer: No
Question: Can these new technologies change my DNA?
Answer: No
When and how long will I be protected by the COVID 19 Vaccine?
Honesty with your staff is the best policy.
- We will most likely not know how long the vaccine will be protective when we receive it, more research needed
- Most of the vaccines are 2 doses, 3-4 weeks apart
- Protection 1-2 weeks after the second dose
- May need to have vaccine shots for COVID-19 on a regular basis (like the flu shot or boosters for children)
Question: Will I still need to wear a mask?
Answer: Yes. COVID is transmitted predominately by respiratory droplets generated when people cough, sneeze, sing, talk, or breathe. CDC recommends community use of masks which reduce the emission of virus-laden droplets, which is especially relevant for asymptomatic or presymptomatic infected wearers who feel well and may be unaware of their infectiousness to others, and who are estimated to account for more than 50% of transmissions. Masks will ensure backup protection against the virus. (source)
Important: warn about possible side effects: Will the vaccine make me sick?
Side effects reported include:
- Short-term discomfort : headache, muscle pains, fatigue, chills, fever and pain at injection site lasting 1-2 days
- Same symptoms as COVID 19 – Emphasize that the vaccine cannot give you COVID-19
- More pronounced with second dose
- Normal and common with all vaccines
- It means your body is doing its job and making antibodies ( IT IS A GOOD THING)
- MUST COME BACK FOR SECOND DOSE, must be the same vaccine as the first dose (different vaccine brands inoculate against the virus in different ways)
Special circumstances: Past COVID 19 infection or tested for antibodies
- It is safe to get the COVID 19 vaccine even if you have had COVID 19
- If + Antibodies PLEASE get the COVID 19 Vaccine
- Monoclonal antibody treatment trial
Reliable resources for COVID vaccine related information
CDC: https://www.cdc.gov/vaccines/hcp/covid-conversations/answering-questions.html
CDC: About COVID-19 vaccines: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/about-vaccines.html
CDC: Provider Resources for COVID-19 Vaccine Conversations with Patients and Answering Patients’ Questions: https://www.cdc.gov/vaccines/hcp/covid-conversations/
![Colorado Health Care Association [logo]](https://www.cohca.org/wp-content/themes/cohca/images/logo.png)